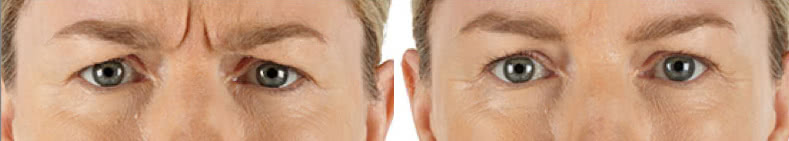
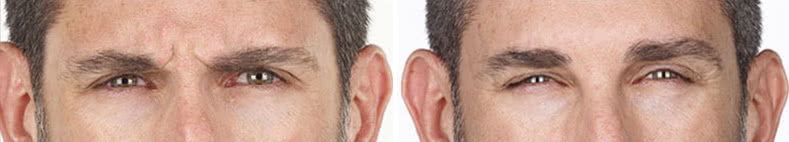
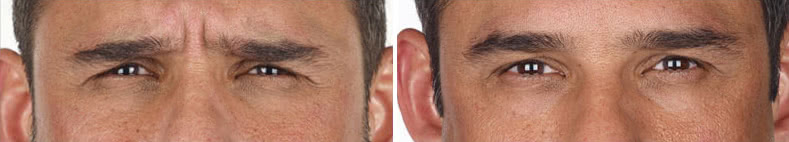
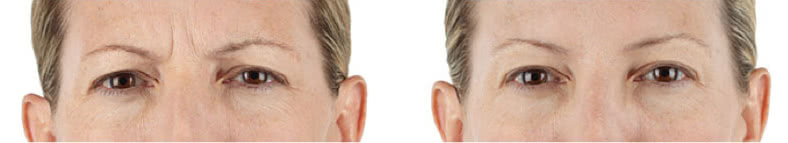
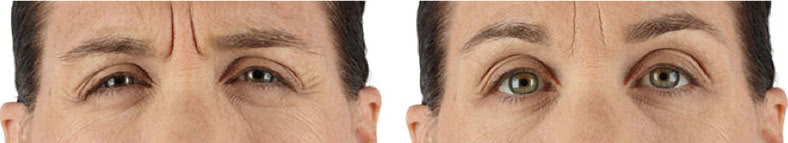
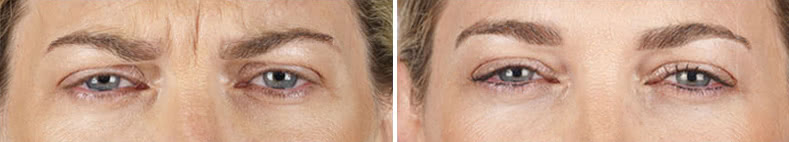
Looking and feeling your best starts here.
Get in touch today for a consultation.

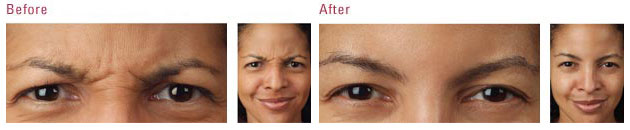
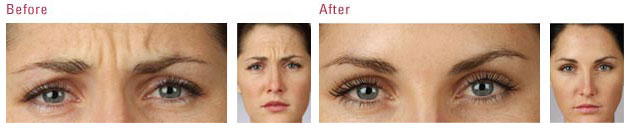
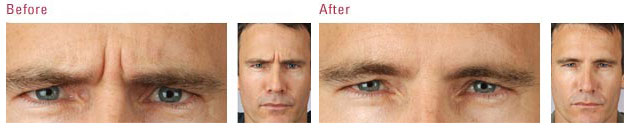
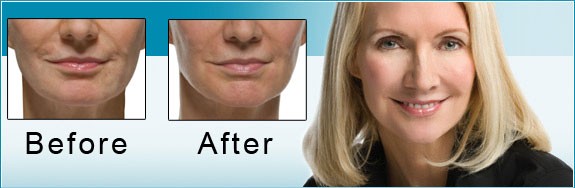
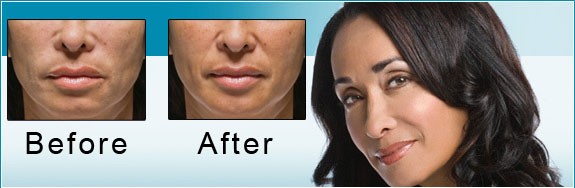








The US Food and Drug Administration (FDA) is the federal governmental agency charged with protecting the health and welfare of the US public by regulating prescription products. FDA regulations are extremely stringent, and when a drug is FDA approved, that means its efficacy and safety have been scientifically studied in valid clinical trials. FDA approval also requires the sponsoring pharmaceutical company to record all adverse events reported after the drug is approved.
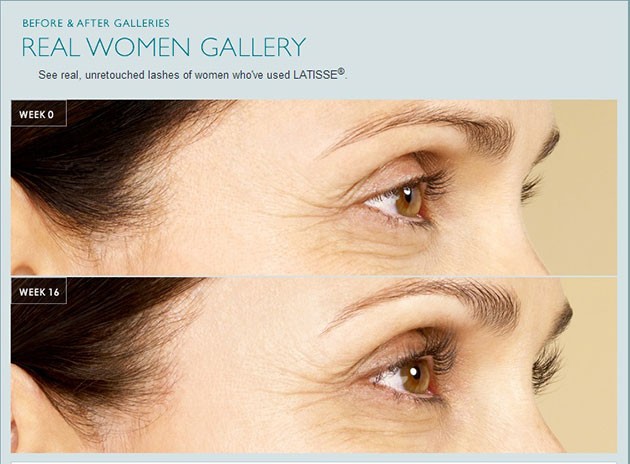
LATISSE® received its FDA approval in December 2008.LATISSE® solution is intended for use on the skin of the upper eyelid margin at the base of the eyelashes. DO NOT APPLY to the lower lid. If patients are using LUMIGAN® (bimatoprost ophthalmic solution) or other products in the same class used for elevated intraocular pressure (IOP), or if they have a history of abnormal IOP, they should only use LATISSE® under the close supervision of their physician.
LATISSE® use may cause darkening of the eyelid skin, which may be reversible. LATISSE® use may also cause increased brown pigmentation of the colored part of the eye, which is likely to be permanent.
It is possible for hair growth to occur in other areas of the skin that LATISSE® frequently touches. Any excess solution outside the upper eyelid margin should be blotted with a tissue or other absorbent material to reduce the chance of this happening. It is also possible for a difference in eyelash length, thickness, fullness, pigmentation, number of eyelash hairs, and/or direction of eyelash growth to occur between eyes. These differences, should they occur, will usually go away if the patient stops using LATISSE® solution.